Barton Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Barton reaction, also known as the Barton nitrite ester reaction, is a photochemical reaction that involves the
 The carbon centered radical can be intercepted by other radical sources such as iodine or
The carbon centered radical can be intercepted by other radical sources such as iodine or


photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of an alkyl nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
to form a δ-nitroso
In organic chemistry, nitroso refers to a functional group in which the nitric oxide () group is attached to an organic moiety. As such, various nitroso groups can be categorized as ''C''-nitroso compounds (e.g., nitrosoalkanes; ), ''S''-nitroso ...
alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
.
Discovered in 1960, the reaction is named for its discoverer, Nobel Laureate Sir Derek Barton
Sir Derek Harold Richard Barton (8 September 1918 – 16 March 1998) was an English organic chemist and Nobel Prize laureate for 1969.
Education and early life
Barton was born in Gravesend, Kent, to William Thomas and Maude Henrietta Barton ( ...
. Barton's Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
in 1969 was awarded for his work on understanding conformations of organic molecules, work which was key to realizing the utility of the Barton Reaction.
The Barton reaction involves a homolytic RO–NO cleavage, followed by δ-hydrogen abstraction
In chemistry, a hydrogen atom abstraction or hydrogen atom transfer (HAT) is any chemical reaction in which a hydrogen free radical (neutral hydrogen atom) is abstracted from a substrate according to the general equation:
:X^\bullet + H-Y -> X-H ...
, free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
recombination, and tautomerization to form an oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted ...
. Selectivity for the δ-hydrogen is a result of the conformation of the 6-membered radical intermediate. Often, the site of hydrogen atom abstraction can be easily predicted. This allows the regio- and stereo-selective introduction of functionality into complicated molecules with high yield. Due to its unique property at the time to change otherwise inert substrates, Barton used this reaction extensively in the 1960s to create a number of unnatural steroid analogues.
While the Barton reaction has not enjoyed the popularity or widespread use of many other organic reactions, together with the mechanistically similar Hofmann–Löffler reaction
The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, i ...
it represents one of the first examples of C-H activation chemistry, a field which is now the topic of much frontline research in industrial and academic chemistry circles.
Preparation of alkyl nitrites
The unusual alkyl nitrite starting material of the Barton reaction is prepared by attack of an alcohol on a nitrosylium cation generated in situ by dehydration of doubly protonated nitrous acid. This series of steps is mechanistically identical to the first half of the mechanism formation of the more well-known aryl and alkyl diazonium salts. While the synthesis of alkyl nitrites fromnitrosyl chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a st ...
is known and oft-employed in the context of complex molecule synthesis, the reaction is reversible and the products are in thermodynamic equilibrium with the starting material. Furthermore, nitrosyl chloride is a powerful oxidizing agent, and oxidation of the alcohols with concomitant chlorination has been observed. The reaction of nitrosyl chloride with aromatic alcohols generally yields nitroso compounds and other over-oxidation products.
Reaction mechanism and regioselectivity
The Barton reaction commences with a photochemically induced cleavage of the nitrite O-N bond, typically using a high pressure mercury lamp. This produces an alkyoxyl radical which immediately abstracts a hydrogen atom from the δ-carbon. In the absence of other radical sources or other proximal reactive groups, the alkyl radical recombines with the nitrosyl radical. The resultant nitroso compounds undergoestautomerization
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
to the isolated oxime product.
acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular ...
. The first instance results in the δ-hydrogen being replaced with iodine, then subsequent cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where al ...
to a tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
by an SN2 reaction
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed in a concerted way, i.e., in one step. The name SN2 refers to the Hughes-Ingold symbol of the m ...
. The second example results in a chain elongation product with the oxime formed 2 carbon units further from the oxygen than normal.
This mechanistic hypothesis is supported by kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for th ...
experiments. Isotopic labeling of the nitrite with 15N has shown the mechanism non-‘caged’ and that the nitrosyl radical formed from a given nitrite recombines randomly with other alkyl radicals. However, recombination of the nitrosyl radical with the alkoxyl radical (a reversal of the homolytic cleavage) has been shown to proceed without scrambling of isotope labels. This lack of tight radical pairing is also supported by the observation that alkyl radicals generated by Barton conditions can undergo radical cyclization while analogous intermediates generated by lead tetraacetate
Lead(IV) acetate or lead tetraacetate is an organometallic compound with chemical formula . It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically store ...
oxidation do not.
In rare cases, it appears that the alkoxyl radical may epimerize before hydrogen atom abstraction.
Most commonly, including steroidal systems, the hydrogen atom is abstracted from a methyl group that has a 1,3 diaxial relationship with the alkoxyl radical. In the absence of a hydrogen on the δ-carbon, or when the particular conformation of the substrate orients the ε-carbon close together, 1,6-hydrogen atom transfer is the favored process. However, these reactions tend to be an order of magnitude slower than the corresponding 1,5-hydrogen atom transfer.
Computational studies have shown that this preference for 1,5-hydrogen atom transfer over 1,6-hydrogen atom transfer appears to be entropically favored rather than a result of a particular stable ‘chair-like’ transition state. In fact, it has been calculated that the 1,6-hydrogen atom transfer proceeds through a transition that is about 0.8 kcal/mol lower than that of the 1,5.
In acyclic systems, δ-hydrogen abstraction is still observed, however, alpha-hydrogen abstraction to form the corresponding ketone competes.
In certain cases, particularly nitrites derived from cyclopentyl alcohols, the oxygen-centered radical prefers to react via C-C bond cleavage as opposed to H-atom abstraction. For example, when subjected to Barton conditions, cyclopentyl nitrite forms glutaraldehyde monoxime. This is also observed in cases where the radical intermediate formed by fragmentation is particularly stable, such as the allylic radical formed by the fragmentation of isopulegol nitrite.
Variants
In rigid systems such as aldosterone, the 1,5-hydrogen atom transfer is exceedingly fast, with a rate constant on the order of 10^7 s-1. Similar intermolecular H-atom transfer can be up to 100 times slower. Furthermore, the hydrogen atom transfer benefits from the formation of a stronger O-H bond at the expense of a weaker C-H bond. For the formation of a primary, second, or tertiary alkyl radical from an alkoxyl radical, there is a driving force of 3 kcal/mol, 5 kcal/mol, and 9 kcal/mol, respectively. The alkyl radical formed after hydrogen atom transfer is susceptible to standard radical reactions when scavengers are present in sufficient excess to outcompete the nitrosyl radical. Soon after their initial disclosure, Barton and co-workers reported the trapping of the radical with I2 and CCl3Br (as Iodine and Bromine radical sources, respectively) to form the δ-halo-alcohol. These halohydrin species can be cyclized to the correspondingtetrahydropyran
Tetrahydropyran (THP) is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. It is named by reference to pyran, which contains two double bonds, and may be produced from it by addin ...
derivates under basic conditions.
Large excesses of activated alkenes can be used to intercept the alkyl radical and results in formation of a C-C bond from an unactivated C-H bond.
In the presence of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, the alkyl radical is trapped and forms an organic peroxy radical. This intermediate is trapped by the nitrosyl radical and then isomerizes to give a δ-nitrate ester which, while both acid- and base-stable, can be reduced to the corresponding alcohol under mild conditions.
Applications in complex molecule synthesis
Aldosterone acetate
In a publication immediately proceeding Barton’s initial disclosure of the methodology in theJournal of the American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical ...
, a synthesis of aldosterone
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a c ...
acetate is demonstrated. Allowing corticosterone acetate to react with nitrosyl chloride in dry pyridine yields the nitrite. Subsequently, irradiation under inert atmosphere followed by treatment with aqueous sodium nitrite selectively gives the desired oxime. The oxime is then acetylated and hydrolyzed to yield the natural product hemiacetal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemike ...
.

Perhydrohistrionicotoxin
After a short synthesis to obtain the desired spiro- .4system, Nobel LaureauteE.J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many ...
and co-workers employed a Barton reaction to selectively introduce an oxime in a 1,3-diaxial position to the nitrite ester. The oxime is converted to a lactam
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek prefixes in alphabetical order indicate ring size:
* α-Lactam (3-atom rings)
* β-Lacta ...
via a Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones ...
and then reduced to the natural product.

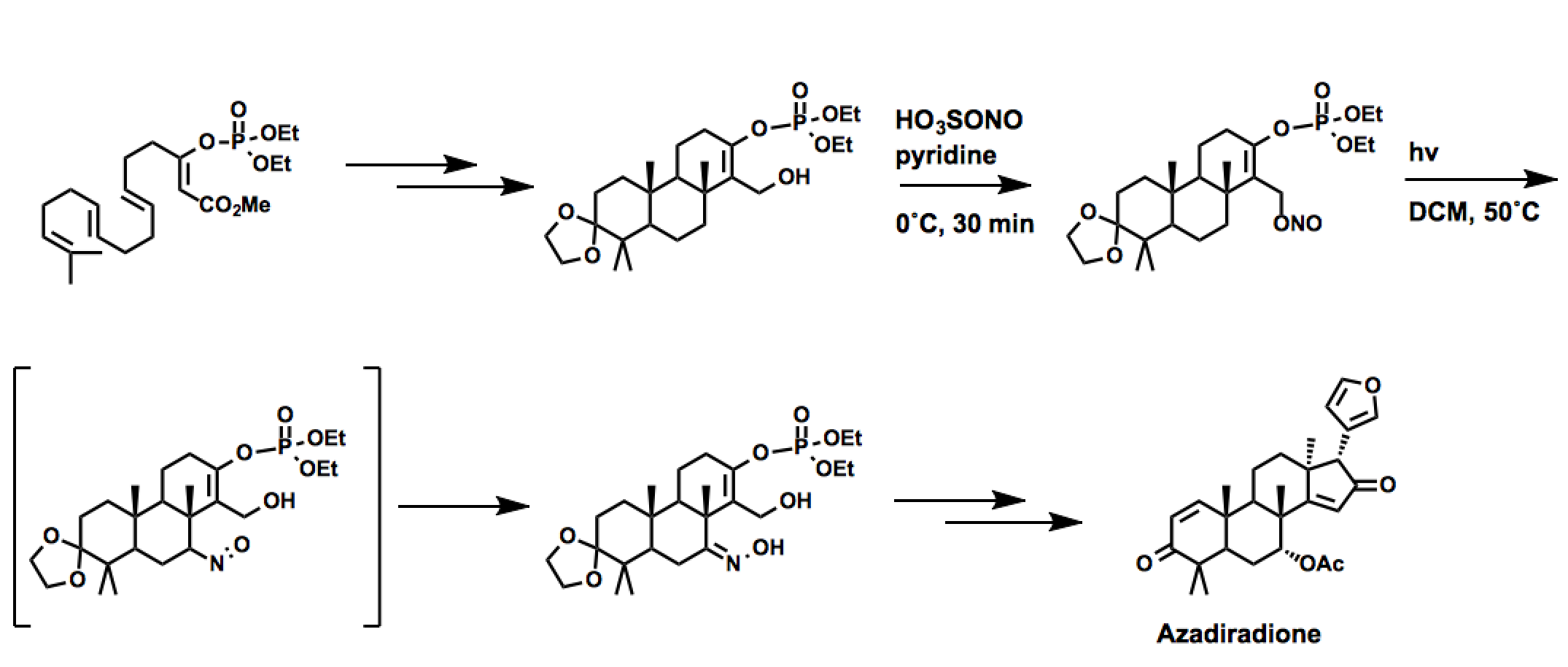
Azadiradione
Corey again employed the Barton reaction in the synthesis of Azadiradione, a member of the limonoid family of natural products. In this case,nitrosylsulfuric acid
Nitrosylsulfuric acid is the chemical compound with the formula . It is a colourless solid that is used industrially in the production of caprolactam, and was formerly part of the lead chamber process for producing sulfuric acid. The compound is t ...
is used in place of nitrosyl chloride.

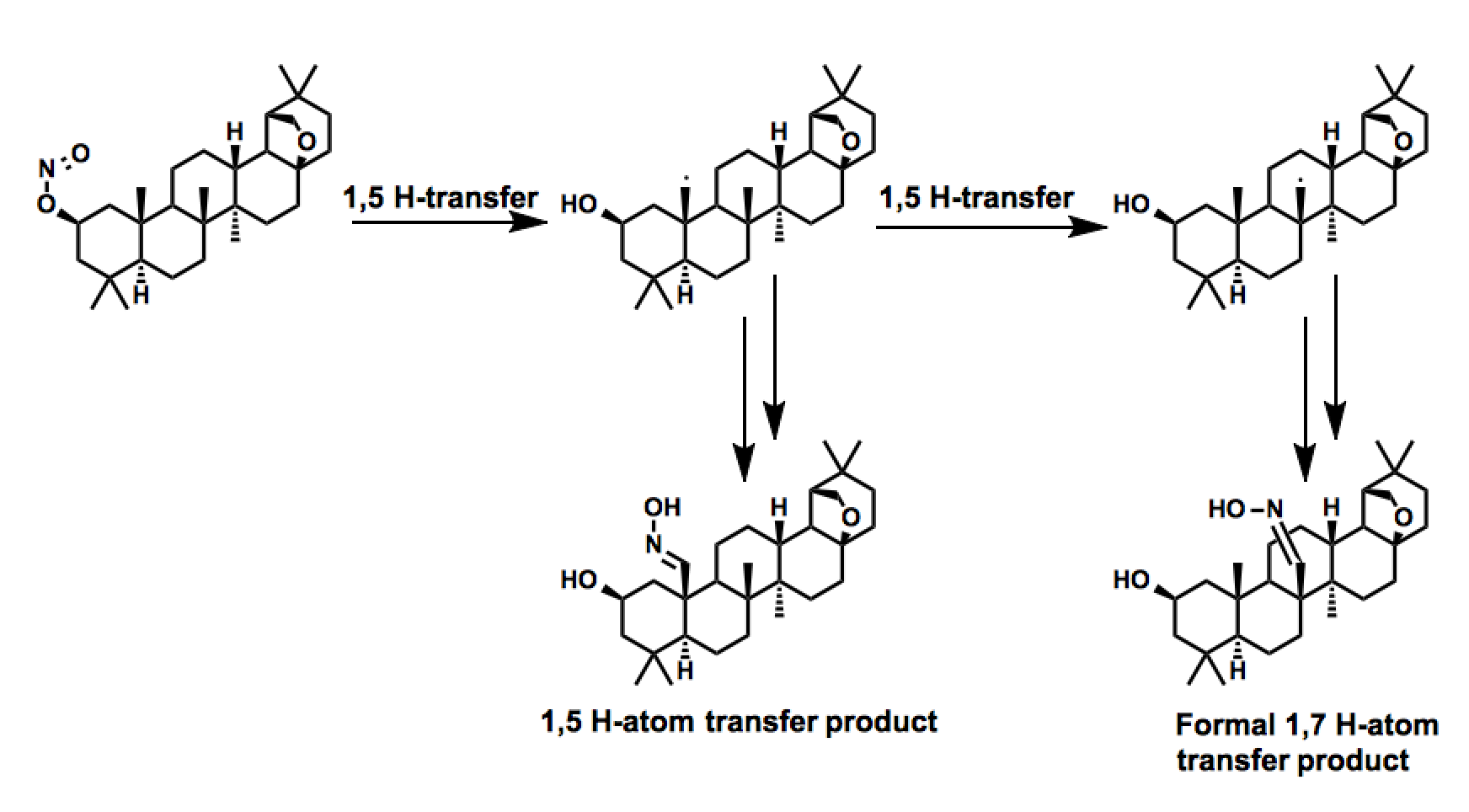
Allobetulin derivatives
In the process of preparing a series of derivatives of the triterpenoid allobetulin, Dehan and coworkers observed a remarkable transformation resulting from two consecutive 1,5-hydrogen atom transfers. While the product of the single 1,5-hydrogen atom transfer was also observed, the former transformation represent a formal 1,7-hydrogen atom transfer across an enormous distance.
References
* László Kürti, Barbara Czakó: ''Strategic Applications of Named Reactions in Organic Synthesis''; Elsevier Academic Press, Burlington-San Diego-London 2005, 1. Edition; . {{Organic reactions Free radical reactions Name reactions